Anna Joselle Lomboy ChE'18 MChE'20 and Professor Robert Q. Topper Publish Paper on Proton Transfer and Hydrogen Bonds Within Aerosol Nanoparticles
POSTED ON: March 18, 2021
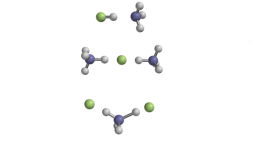
Image Description - Top: Traditional hydrogen bonding between hydrogen fluoride and ammonia. Middle: Proton-shared hydrogen bonding within an anion cluster. Bottom: proton-shared and traditional hydrogen bonding within a cation cluster.
Since 2017, Anna Joselle Lomboy ChE’18 MChE’20 and Professor of Chemistry Robert Q. Topper, have been working together on projects related to proton transfer between acids and bases within tiny particles formed within aerosols, with applications to atmospheric chemistry in polluted environments.
For Lomboy’s senior year, she and Prof. Topper tackled a particularly difficult problem – proton transfer within clusters of molecules composed of a weak acid (hydrogen fluoride) and a weak base (ammonia). Using computational quantum mechanics software and high-performance computing platforms, they have completed and released a study entitled “Nonuniform Proton Transfer and Strong Hydrogen Bonding within Cation, Anion, and Neutral Clusters of Ammonia and Hydrogen Fluoride” which has been accepted for publication in the Journal of Physical Chemistry A, March 18, 202, Volume 125, Issue 10.
The onset of COVID-19 restrictions made the technical aspects of the completion of this research extremely difficult. In their paper, Lomboy and Professor Topper acknowledged Keith Ng and Wayne Adams of The Jeanette and Louis Brooks Computer Center at The Cooper Union, for their invaluable support and assistance throughout the project and especially during the past year. In addition, the authors emphasized that the framework used for analysis was built from ideas developed by Steve Scheiner (Utah State), Janet E. Del Bene (Youngstown State), Hans-Heinrich Limbach (Freie Universitat Berlin), and their collaborators.
Lomboy and Professor Topper’s work not only provides fundamental information for atmospheric chemistry, but also yields insights into the fundamental of hydrogen bonding and its relationship to proton transfer. The reasoning is, within these particles proton transfer is incomplete, yielding three kinds of hydrogen bonding within one type of acid-base system depending on the size and ionic charge of the nanoparticle. As Professor Topper jokingly puts it, “Sometimes a salty nanoparticle is only sort of salty.”
Anna Joselle Lomboy is currently a Senior Scientist at Henkel AG & Co.




